Motor ability, as a core product of animal evolution, is an adaptive survival skill essential for sustaining life. The integrity of the motor system directly determines an individual’s success in food chain competition, predator avoidance, and access to reproductive opportunities. In vertebrates, the cadherin FAT family comprises a group of single-pass transmembrane cell adhesion molecules, consisting of four members: FAT1–4. Each FAT molecule possesses over 30 extracellular cadherin repeat domains along with related functional domains. Clinical studies have shown that mutations in the FAT2 gene are associated with motor coordination disorders and autism. Due to the large molecular weight and complex structure of these proteins, research on their functional mechanisms has long been hindered. Therefore, elucidating the molecular mechanism by which FAT2 regulates motor behavior provides a critical breakthrough for understanding the pathogenesis of motor disorders.
Recently, Bo Zhang lab published an article titled "Atypical cadherin FAT2 is required for synaptic integrity and motor behaviors" in the famous neuroscience journal Journal of Neuroscience. The study elucidates the physiological function of FAT2, which is specifically expressed in cerebellar granule cells, and demonstrates that FAT2 is crucial for synaptic integrity as well as the execution of fine motor skills and digging behaviors.
Previous research from the Mishina group and the Yuzaki group in Japan found that the synaptic bridging protein Cbln1 regulates parallel fiber synaptic function by connecting the presynaptic membrane protein Neurexin with the postsynaptic membrane protein GluD2. The Mishina group also discovered that FAT2 co-purifies with Cbln1 in cerebellar granule cells, Liqun Luo lab fromStanford University found that FAT2 is enriched on the surface of Purkinje cells. These findings suggest that FAT2 may be involved in the regulatory complex of Neurexin/Cbln1/GluD2. However, the specific mechanism of FAT2's role in the central nervous system remains unclear.
The authors first employed cell surface binding and cell aggregation assays, which revealed that FAT2 directly binds to Cbln1. This interaction was found to be dependent on the first two cadherin repeat domains of FAT2. Furthermore, the presence of presynaptic Neurexin completely blocked this interaction (Figure 1), indicating that Neurexin has a significantly higher binding affinity for Cbln1 compared to FAT2.

Figure 1: FAT2 is a binding partner of Cbln1
Using RNAscope in situ hybridization, the authors confirmed that FAT2 mRNA is specifically enriched in cerebellar granule cells. To study its function, they utilized FAT2 conditional knockout (cKO) mice. They found that the specific deletion of FAT2 in granule cells did not alter the overall macroscopic structure of the cerebellum. Furthermore, it did not affect the axon growth of granule cells or the morphology of Purkinje cells.The authors then examined the effect of FAT2 knockout on synaptic structure and discovered that the deletion of FAT2 decreased the density of vGluT1 (a marker for parallel fiber presynaptic terminals) while increasing the density of vGluT2 (a marker for climbing fiber presynaptic terminals). Electrophysiological recordings further showed a significant reduction in the amplitude of excitatory postsynaptic currents at parallel fiber synapses. Interestingly, FAT2 knockout led to a synchronous enhancement in both the frequency and amplitude of inhibitory synaptic currents in Purkinje cells, a phenotype similar to that observed in Cbln1-deficient models (Figure 2). Given the interaction between FAT2 and Cbln1, these results suggest that FAT2 may influence inhibitory synaptic plasticity indirectly by regulating Cbln1.
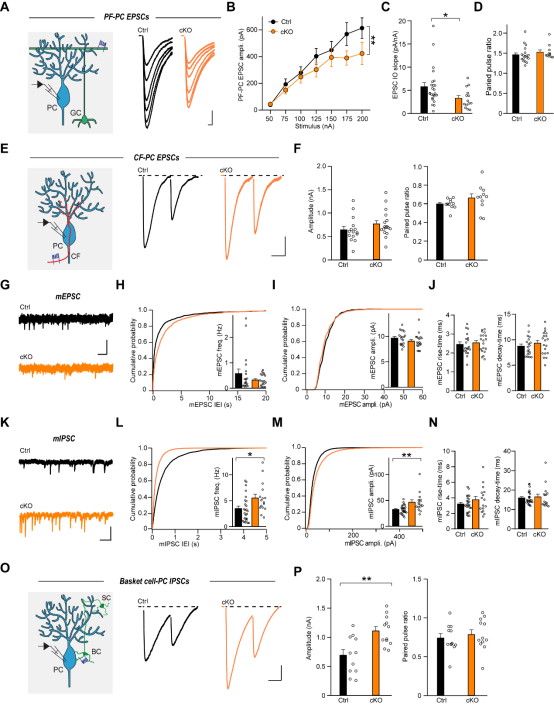
Figure 2: FAT2 deficiency reduces parallel fiber synaptic transmission
The authors then conducted systematic behavioral tests on FAT2 knockout mice and found that the FAT2 knockout mice exhibited deficits in challenging motor tasks—they took significantly longer to traverse a 10mm cylindrical balance beam, showed impaired motor coordination only during the high-speed phase of the accelerated rotarod test, and displayed reduced marble-burying behavior. However, their basic motor abilities and performance in fine forelimb grasping tasks remained normal. Behavioral assessments such as the open field test, elevated plus maze, and Y-maze confirmed that FAT2 knockout did not affect cognitive, emotional, or social functions, indicating that FAT2 specifically regulates cerebellar-dependent complex motor coordination circuits rather than causing generalized neurological dysfunction.
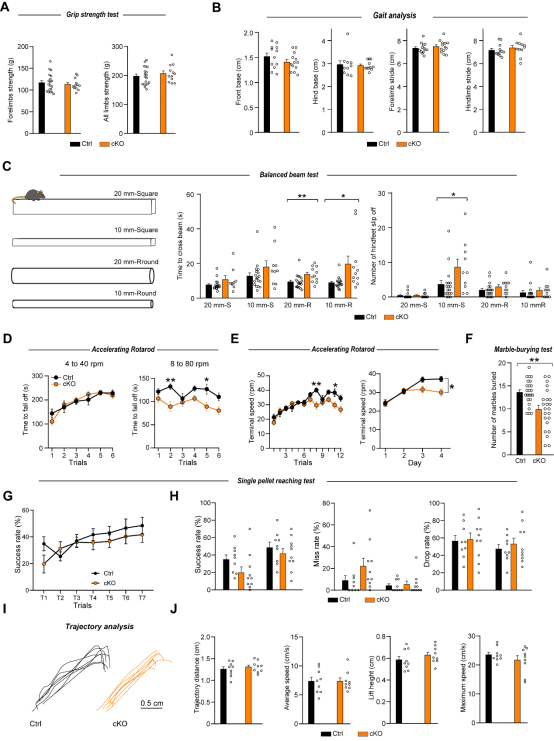
Figure 3. FAT2 is essential for fine motor coordination
The marble-burying test suggested impairments in digging behavior. The authors then performed a detailed analysis of digging behavior in FAT2 knockout mice during the marble-burying test. They found that FAT2 knockout significantly impaired refined digging behavior—specifically, the frequency of Type 1 (digging/alternating forepaws) movements decreased, and the durations of both stereotyped digging (single bouts >8 seconds) and non-stereotyped digging were reduced (Figure 4). Notably, this deficit was behavior-specific:analyzesof resting gait and self-grooming duration were normal, confirming that FAT2 deficiency selectively impairs the execution of cerebellar-dependent refined motor programs rather than affecting basic motor function or altering repetitive behavioral patterns.
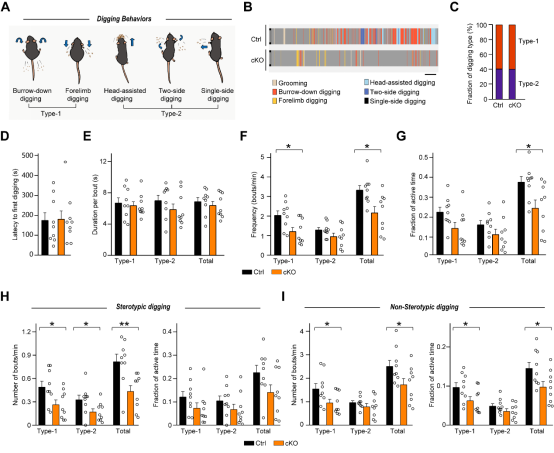
Figure 4. FAT2 is essential for the regulation of digging behavior
This study is the first to reveal that FAT2 specifically binds to the synaptic regulatory protein Cbln1 through its first two cadherin repeat domains (Figure 5). FAT2 conditional knockout mice exhibited significant impairments in fine motor coordination and digging behavior while maintaining normal basic motor abilities, underscoring the central role of FAT2 in cerebellar-dependent complex motor regulation. Given that motor dysfunction is a core phenotype of various neuropsychiatric disorders, this discovery provides a new direction for understanding the molecular mechanisms of related diseases and developing targeted therapeutic strategies. Although learned digging relies on broader learning systems, the neural circuitry underlying the fine motor coordination deficits in FAT2 knockout mice remains unclear. The observed alterations in synaptic integrity at parallel fibers, climbing fibers, and inhibitory synapses in FAT2 knockout mice may be related to the deficits in fine motor coordination and natural digging behavior. Furthermore, additional research is needed to determine whether the impairment in digging behavior directly stems from these motor coordination deficits or involves other mechanisms.
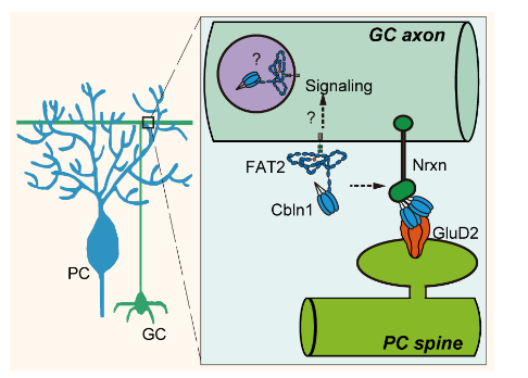
Figure 5. Schematic diagram of FAT2's mechanism of action in parallel fibers
Paper link:https://www.jneurosci.org/content/45/24/e2345242025.long Atypical cadherin FAT2 is required for synaptic integrity and motor behaviors | Journal of Neuroscience
The PhD student XiankunWang in Bo Zhang lab is the first author of the paper. Research assistants Yadi Puand Dr.Jifei Miao (currently working at Shenzhen Bao'an Traditional Chinese Medicine Hospital) are co-first authors of the paper, and investigator Bo Zhang is the corresponding author.Research assistant Li Xie,PhD students Jun Wang, Yongfei Cui, and Ying Han, Dr. Liming Qin in Bo Zhang lab, Liangyu Guan from the Shenzhen Bay Laboratory Translational Innovation Center, and Professor Markus Wöhr from KU Leuven in Belgium also made important contributions to this study. This research was supported by funding from the National Natural Science Foundation of China, Shenzhen Bay Laboratory Major Projects, the Shenzhen-Hong Kong Institute of Brain Science, and other fund projects.
Bo Zhang lab(www.bozhanglab.org) utilizes cutting-edge neuroscience research methods to deeply investigate the molecular mechanisms of neural synapse formation and function, as well as the role of synaptic damage in neuropsychiatric disorders. The research group currently focuses on two main areas:
1)Molecular Mechanisms of Synapse Formation and Functional Maintenance: Systematic research on cell adhesion molecules has been published in several high-impact journals with the principal investigator as the last or sole corresponding author, including Molecular Psychiatry(2017, 2024),Science Advances(2022),Journal of Experimental Medicine(2018), and Journal of Neuroscience(2016, 2025). This research is supported by projects such as the National Natural Science Foundation–International Cooperation Program, National Natural Science Foundation–Excellent Young Scientists Program, National Natural Science Foundation–General Program, and Shenzhen Medical Research Special Fund.
2)Drug Intervention Targeting Core Symptoms of Neuropsychiatric Disorders:Our labis exploring multiple avenues in this direction and looks forward to sharing research outcomes with the neuroscience community.
We sincerely invite and welcome dedicated researchers (Assistant Researchers/Postdoctoral Fellows/Doctoral Students) passionate about molecular neurobiology to join our research group.





